French drugmaker Sanofi and its British partner GlaxoSmithKline are seeking regulatory approval for their COVID-19 vaccine to be used as a booster, as well as a standalone two-dose shot, after several setbacks.
The companies said on Wednesday they intended to submit data to regulators from a late-stage trial of the vaccine, and another testing it as a booster, with full results for both studies expected to be published “later this year.”
Sanofi is hoping for a comeback after falling behind in the race for COVID-19 shots, while GSK, the world’s biggest vaccine maker by sales, has not developed its own candidate and is instead supplying its adjuvant technology to developers.
The two businesses have partnered to produce a pandemic adjuvant from GSK and a recombinant antigen from Sanofi. Both companies have built vaccination platforms that have demonstrated efficacy against influenza.
Our state-of-the-art, global healthcare organization is committed to improving people’s lives by utilizing the miracles of science. Our team, which is dispersed over nearly 100 countries, is dedicated to making the seemingly impossible feasible in an effort to transform the medical field. We provide potentially life-saving medical treatments and vaccine protection to millions of people globally, all while keeping sustainability and social responsibility at the center of our mission. Sanofi is traded on EURONEXT: SAN and NASDAQ: SNY.
GSK cautions investors that there are risks and uncertainties that might cause actual results to differ materially from those anticipated when relying on any forward-looking statements or predictions made by the business, including the ones included in this communication. Some of these variables are listed in the Company’s 2020 Annual Report on Form 20-F, the Q4 results from GSK in 2021, and any COVID-19 pandemic effects, but they are not the only ones.
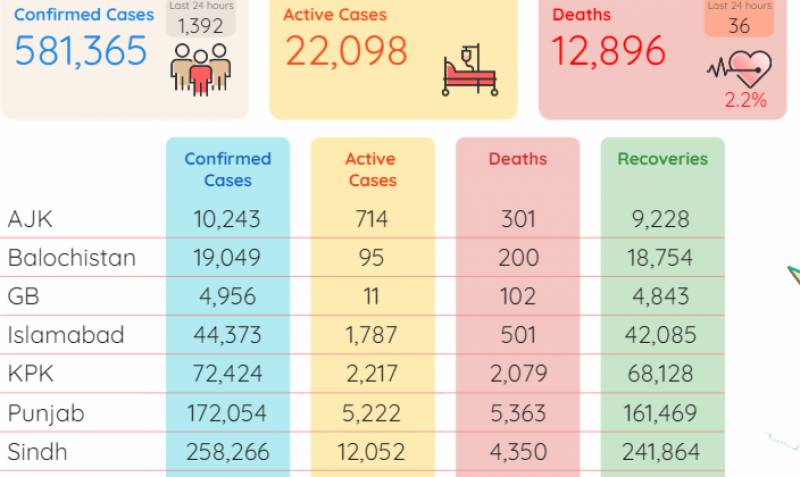
Also Read: COVID-19 POSITIVE COUNTRY DROP TO 2.84%